Controlled Substances Inventory Log 2020-2025 free printable template
Show details
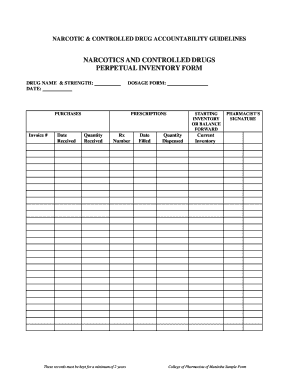
CONTROLLED SUBSTANCES INVENTORY LOG Name of Pharmacy: Address: City: State: Zip Code: Name of REGISTRANT on DEA Registration: DEA Registration Number: Date of Inventory: Inventory Taken At:Opening
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign Controlled Substances Inventory Log
Edit your Controlled Substances Inventory Log form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your Controlled Substances Inventory Log form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit Controlled Substances Inventory Log online
To use the professional PDF editor, follow these steps below:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit Controlled Substances Inventory Log. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out Controlled Substances Inventory Log
How to fill out Controlled Substances Inventory Log
01
Gather all necessary controlled substances that need to be logged.
02
Ensure you have the Controlled Substances Inventory Log form ready.
03
Start by entering the date of the inventory at the top of the log.
04
List each controlled substance name in the corresponding column.
05
Record the quantity of each substance on hand in the quantity column.
06
Note the unit of measure (e.g., tablets, milliliters) for each entry.
07
Include the location of each substance in the designated column.
08
Sign and date the log at the end to confirm its accuracy.
Who needs Controlled Substances Inventory Log?
01
Pharmacies that handle controlled substances.
02
Hospitals and clinics with pharmacy services.
03
Research facilities that use controlled substances.
04
Veterinarians prescribing or administering controlled substances.
05
Any organization required to maintain compliance with state and federal regulations regarding controlled substances.
Fill
form
: Try Risk Free
People Also Ask about
How often should inventory be done on controlled substances?
After the initial inventory is taken, the registrant shall take a new inventory of all stocks of controlled substances on hand at least every two years. The biennial inventory may be taken on any date which is within two years of the previous biennial inventory date.
Is Gabapentin a CII?
Gabapentin isn't considered a controlled substance by the federal government as of July 2022.
How often should controlled drugs be counted?
A check of all CDs stocked within wards and departments must be completed every 3 months by Pharmacy (either a pharmacist or pharmacy technician).
How often is a controlled substance inventory in California?
(c) A pharmacy or clinic shall compile an inventory reconciliation report of all federal Schedule II controlled substances at least every three months.
What is a CII prescription?
CII drugs, also known as Schedule II substances, are those drugs that require additional care because of the potential for the patient to intentionally or unintentionally abuse the drug. Because of that added risk, CII drugs have more regulations, procedures, and laws surrounding them.
How often do pharmacies do inventory?
Frequency. ing to the DEA, pharmacies must conduct an initial inventory of their controlled substances when they open and every two years after. The pharmacy does not have to conduct inventory at a specific date or time as long as the pharmacy meets two-year deadline.
How often must inventory be performed on controlled substances?
After the initial inventory is taken, the registrant shall take a new inventory of all stocks of controlled substances on hand at least every two years. The biennial inventory may be taken on any date which is within two years of the previous biennial inventory date.
How long after a prescription is written does it expire?
When your healthcare provider sends in a prescription to your pharmacy, you usually have up to one year to fill the prescription before it expires in most states. The exception to this is prescriptions for controlled substances, which may only be valid for 6 months or less, depending on state laws.
How often is a pharmacy required to conduct a controlled substance inventory in the state of Michigan?
The State of Michigan requires a physical inventory of all controlled substances to be conducted on an annual basis. The annual inventory must be performed between April 1 and June 30 of each year per Michigan Public Health Code, Section 333.7321.
What does CII on a label mean?
What is a CII drug? CII drugs, also known as Schedule II substances, are those drugs that require additional care because of the potential for the patient to intentionally or unintentionally abuse the drug. Because of that added risk, CII drugs have more regulations, procedures, and laws surrounding them.
How long does a controlled drug prescription last?
Controlled medicines These medicines are sometimes misused, so strict legal controls apply to their supply. A prescription for a controlled medicine is valid for 28 days from the date on the prescription.
Where is the generic name on a drug label?
1:56 14:15 How to Read a Medication Label Nursing Skill - YouTube YouTube Start of suggested clip End of suggested clip And spell than the generic name and the first letter is capitalized. And it's usually the largestMoreAnd spell than the generic name and the first letter is capitalized. And it's usually the largest and boldest now typically found very close by that brand name usually under it is the generic. Name
How often are inventory forms required to be completed for Schedule II drugs?
In addition, the Controlled Substances Act requires that an inventory of controlled substances in a pharmacy be conducted initially (ie, when a DEA registration has been issued) and biennially (ie, every 2 years) thereafter.
How do you know if a drug is a controlled substance by the label?
If a drug is a controlled substance, that fact is indicated on the label with a C and the Roman numeral indicating the drug's schedule category (for example, CII, CIII, CIV, or CV).
What are the requirements when checking in CIII V products quizlet?
CIII-V checked withing 12 hours of receipt. Individuals checking in the controlled substances will sign and date the invoice and document in a prominent location on the face of the documentation that all controlled subtances have been received.
How long is a CII prescription good for in Nevada?
The prescription must be received within 14 days of the “do not fill until” date to fall within the 14-day rule; The date indicated by the practitioner must not be later than 3 months after the date on which the prescription is written.
What is a Schedule 2 controlled substance in Nevada?
Schedule II Controlled Substance Criteria Examples of Schedule II drugs include cocaine, codeine, hydrocodone, morphine, oxycodone, opium, barbiturates, and Ritalin.
What are the requirements when checking CII products?
CII log must be reconciled withing 12 hours of product receipt. CIII-V checked withing 12 hours of receipt. Individuals checking in the controlled substances will sign and date the invoice and document in a prominent location on the face of the documentation that all controlled subtances have been received.
How often must pharmacy inventories be performed Texas?
(c) Annual inventory. (1) A Class A, Class A-S, Class C, Class C-S, or Class F pharmacy shall take an inventory on May 1 of each year, or on the pharmacy's regular general physical inventory date.
Do written prescriptions expire Nevada?
"Do Not FILL UNTIL …. " ALL prescriptions for controlled substances are no longer valid after six months from the date written. NRS 639.2393(1).
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is cii inventory 1?
Cii Inventory 1 is an inventory management system developed by Cii Solutions, Inc. It is designed to help businesses track and manage their inventory more efficiently. Features include inventory tracking, ordering, receiving, and shipping. It also provides tools to help with forecasting, pricing, and reporting.
Who is required to file cii inventory 1?
The Federal Acquisition Regulation (FAR) requires all federal contractors and subcontractors to file a CII Inventory 1. This includes any organization that enters into a contract with the U.S. government or is a subcontractor for a federal contract.
What is the purpose of cii inventory 1?
Cii Inventory 1 is a web-based inventory management system designed to help organizations track their stock levels, monitor product movement, and manage their inventory operations. It enables users to view their stock levels, update inventory records, generate reports, and manage inventory transactions. The system can also be used to forecast demand and manage supplier relationships.
How to fill out cii inventory 1?
To fill out a CII (Controlled Inventory Item) inventory form, follow these steps:
1. Start by entering the name of the entity or person who is responsible for maintaining the inventory.
2. Provide the date on which the inventory is being filled out.
3. List the CII items in separate rows or sections. Each row should include the following details:
a. Description: Provide a brief description of the CII item, including any relevant specifications or details.
b. Manufacturer: Specify the name of the manufacturer or brand associated with the item.
c. Serial/Unique Number: Enter the serial or unique number associated with the CII item, if applicable.
d. Quantity: Indicate the number of CII items of the same type that are being inventoried.
e. Location: Specify the precise location where the CII item is stored or maintained.
f. Custodian/Responsible Person: Enter the name of the person or department responsible for the CII item.
4. Include any additional columns or sections that may be required by your organization's inventory management system or specific needs. This could include columns for purchase date, cost, expiration date, etc.
5. Add a section for comments or notes, where any relevant information or observations regarding the CII items can be recorded.
6. Finally, review the completed inventory form for accuracy and completeness before saving, printing, or submitting it as required.
Note: The format or specific fields in a CII inventory form may vary depending on the organization's requirements or industry regulations. It is essential to adjust the form accordingly and refer to any guidelines or instructions provided.
How do I complete Controlled Substances Inventory Log online?
Easy online Controlled Substances Inventory Log completion using pdfFiller. Also, it allows you to legally eSign your form and change original PDF material. Create a free account and manage documents online.
How do I fill out the Controlled Substances Inventory Log form on my smartphone?
Use the pdfFiller mobile app to complete and sign Controlled Substances Inventory Log on your mobile device. Visit our web page (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, the capabilities you’ll have access to, and the steps to take to get up and running.
How do I fill out Controlled Substances Inventory Log on an Android device?
Complete Controlled Substances Inventory Log and other documents on your Android device with the pdfFiller app. The software allows you to modify information, eSign, annotate, and share files. You may view your papers from anywhere with an internet connection.
What is Controlled Substances Inventory Log?
The Controlled Substances Inventory Log is a detailed record-keeping document used to track and monitor the quantity and distribution of controlled substances within a facility, ensuring compliance with regulatory requirements.
Who is required to file Controlled Substances Inventory Log?
Pharmacies, hospitals, and any healthcare facilities that handle controlled substances are required to file the Controlled Substances Inventory Log as part of their compliance with state and federal regulations.
How to fill out Controlled Substances Inventory Log?
To fill out the Controlled Substances Inventory Log, ensure that all relevant information such as the date, type of substance, quantity on hand, and location of storage are accurately documented. Each entry should be signed and dated by the person conducting the inventory.
What is the purpose of Controlled Substances Inventory Log?
The purpose of the Controlled Substances Inventory Log is to maintain an accurate account of controlled substances, prevent diversion or theft, and ensure compliance with legal regulations governing their use and distribution.
What information must be reported on Controlled Substances Inventory Log?
The Controlled Substances Inventory Log must report information such as the name of the controlled substance, the quantity on hand, the date of the inventory, the location of storage, and signatures of the individuals conducting the inventory.
Fill out your Controlled Substances Inventory Log online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Controlled Substances Inventory Log is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.